

Therefore, we hypothesize that USPIO nanoparticles, especially ≤10 nm, have significant potential as positive contrast agents. Faster biodegradation rates in the liver and spleen have been recently reported for monodisperse 5 nm iron oxide cores in comparison with 15 and 30 nm iron oxide cores coated with the same coating molecules.

Thirdly, USPIO nanoparticles with smaller diameters have shown better biosafety. Furthermore, USPIO enhancement effect on T 1 relaxation rate is stronger with decreased particle size and core size (∼5 nm) has been suggested to be optimal positive ( T 1) contrast agent benefit from the enhancement of T 1 and suppression of T 2. Secondly, unlike most SSPIO nanoparticles, which predominantly enhance the transverse relaxation rate (1/ T 2) and function as negative contrast agents, some USPIO nanoparticles with smaller core size can enhance both longitudinal (1/ T 1) and transverse relaxation rates. First, larger SPIO nanoparticles show higher nonspecific uptake by the mononuclear phagocyte system (MPS) or reticuloendothelial system (RES) compared with smaller USPIO nanoparticles, which indicates a higher percentage of passive uptake of larger particles for tissues rich in macrophages, such as the liver, spleen, lymph nodes, or bone marrow. Some differences between SSPIO and USPIO nanoparticles have been reported. SPIO nanoparticles are typically classified by their hydrodynamic diameter into three categories, which are oral (large) SPIO nanoparticles at 300 nm to 3.5 μm, standard (regular) SPIO (SSPIO) nanoparticles at 50 to 150 nm, and ultrasmall SPIO (USPIO) nanoparticles less than 50 nm. In addition, the low cost, biological safety, and flexible surface modifications promote wide utilization of SPIO nanoparticles as contrast agents or as a platform for construction of specific targeting probes in MRI research.
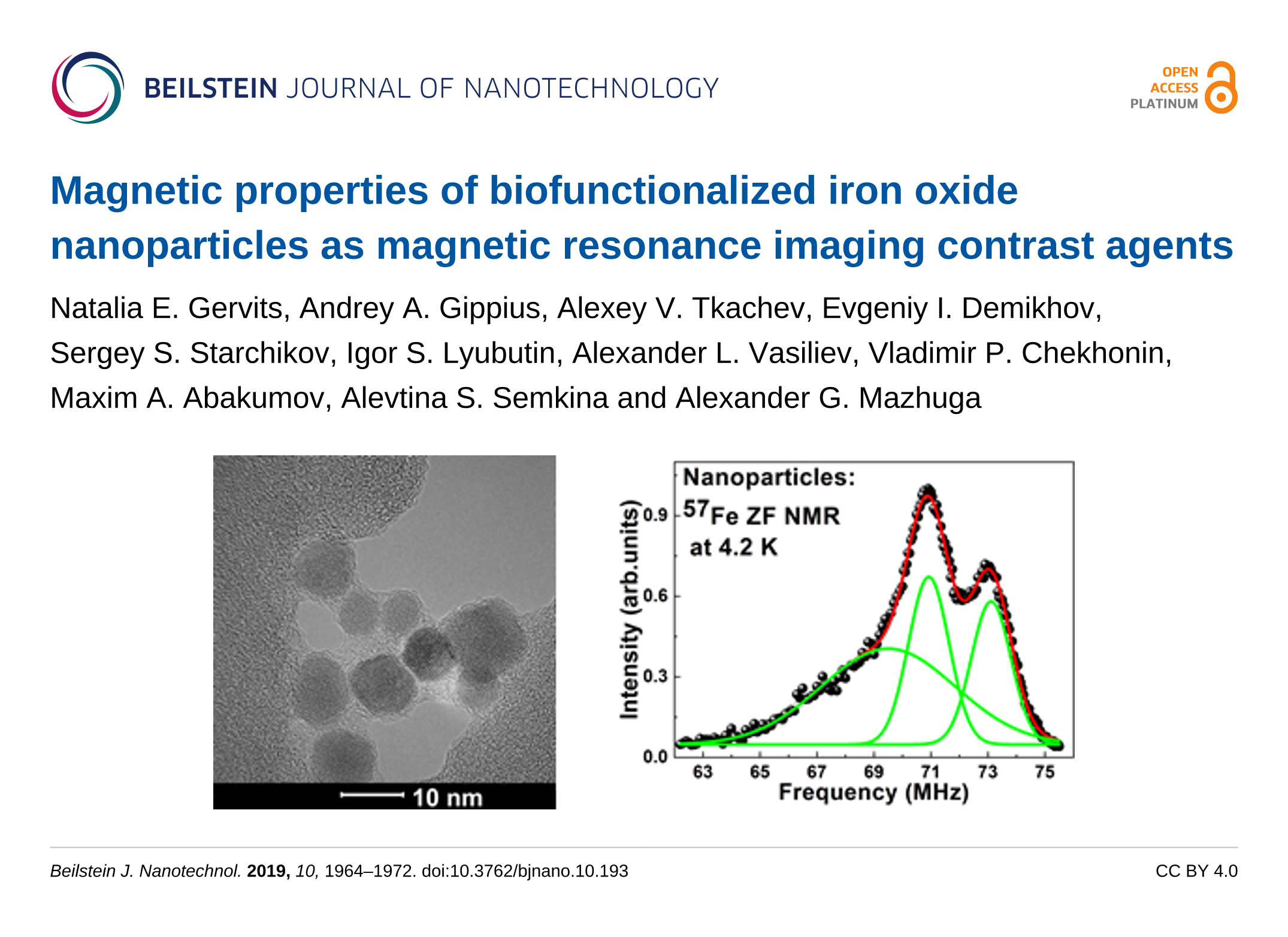
In contrast from gadolinium-based contrast agents, SPIO nanoparticles showed stronger magnetic susceptibility and size- or surface-dependent pharmacokinetics and image features. They are attracting intensive attention and have many potential applications in MRI because of their novel magnetic properties and surface chemistry for ligand binding and biosafety optimization. SPIO nanoparticles have been approved by the U S Food and Drug Administration (FDA) and European Commission for use as a type of MRI contrast agent. Among diverse MR molecular imaging studies, superparamagnetic iron oxide (SPIO) nanoparticles have played an important role. It is also emerging as a kind of functional MR probe system, for example, stimuli-responsive MRI-monitored drug delivery system, including pH-responsive or thermo-responsive ones. The latest results of molecular imaging using MR scanning provide new methods for noninvasive detection or tracking of such conditions as hepatocellular carcinoma, atherosclerotic plaque, collateral circulation in acute ischemic stroke, and glioma gene therapy. Magnetic resonance imaging (MRI) is widely used in clinical practice due to its advantages of nonionizing radiation, multisequencing, and better soft tissue contrast. Taken together, these data might inspire a new personalized and precise diagnostic tool and stimulate new applications for specific targeted molecular probes. Furthermore, USPIO nanoparticles showed relatively good in vitro biocompatibility and fast clearance (within 45.17 minutes after intravenous injection) in the normal liver. In addition, USPIO nanoparticles (<5 nm) in vivo demonstrated both positive ( T 1) and negative ( T 2) liver contrast enhancement in healthy rats’ liver. The results showed that USPIO nanoparticles (<5 nm) could potentially reduce longitudinal and transverse relaxation times and showed similar T 1 relaxation rates compared with commercial gadolinium chelates. In this study, we used MRI to study the in vitro phantom and in vivo rat liver imaging characteristics of USPIO nanoparticles (<5 nm). However, there is still a lack of MR imaging studies on the liver assessing USPIO nanoparticles <5 nm in size to reflect its absorption and clearance properties. The smaller size and prolongation of the plasma half-life change the in vivo fate of ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles captured by liver in reticuloendothelial system (RES) or mononuclear phagocytic system (MPS). They provide new possibilities for noninvasive diagnosis and treatment monitoring, especially for tissues that are rich in macrophages. Iron nanoparticles have an increasingly more and more important role in MR molecular imaging due to their novel magnetic and surface chemical properties.


 0 kommentar(er)
0 kommentar(er)
